| Insect Behaviour |
For sexual and parental behaviour in insects read the insect life-cycles article.
Choice Experiments and Chemotaxis
Above: a wide range of devices have been employed to observe insect behaviour in the laboratory. A
simple arena, consisting of a petri dish may have odour sources, for example, place din the centre. A
perspex box could be used to study the preference of flies to land on certain objects placed within it.
Olfactometers are used to measure the response of an insect to an odour by giving it a choice of
odours. Typically several inlets carry streams of air into the chamber and one or more of these streams
may contain an introduced odour and the insect is then free to move towards or away from the odour or
to behave indifferently to it. A Y-tube choice-chamber provides a convenient set-up in many cases,
with two air streams entering the chamber through guaze meshes (which screen out visual cues and
prevent the insect from escaping. Care is always needed in these experiments. Some insects leave
chemical trails that can be detected by their fellows and this may bias a Y-tube choice experiment, for
example, if the chamber is not cleaned or replaced between trials and insects prefer to follow the paths
of the previous test subject. Insects also have memory, using the same individual repeatedly can bias
results, for example if an insect turned left in a Y-tube and this led to its release from the apparatus,
then it might remember that left leads to escape and prefer to keep turning left on subsequent trials.
Insects make good subjects for the study of animal behaviour. Their nervous systems and behaviours
may be complex, but it is possible to discern mechanisms and rules governing insect behaviour. They
are also generally readily available in large numbers and easy to manipulate in the laboratory.
In this article, we are going to look at the petri-dish arena. The floor of the arena will be covered with
filter paper, which the insects can easily grip and walk across and which can be replaced for each trial,
eliminating any trails that may be deposited. The insects will be filmed under even infra-red illumination,
which most insects can not see, thus eliminating responses to remote light sources, but which we can
detect with an infra-red camera, enabling us to film and record the insects' behaviour. A small drop of
odourant can be added to the centre of the arena, onto the filter paper (or a separate filter-paper disc)
and then an insect added to the arena some distance from the source and its behaviour recorded.
The patterns of movement obtained from such an experiment may be hard to analyses, compared to
say a simpler Y-tube choice experiment. Parameters like how long the insect spends near the source, or
how many times it walks towards it can be measured. However, with computer modeling more complex
analysis is possible.
First of all, we need a control. We should observe a number of insects in the arena in the absence of an
odour source (and also in the presence of just the solvent used to dissolve the odour source, such as
water or paraffin oil). Typically, in odourless controls the following pattern of movement is seen:
Choice Experiments and Chemotaxis
Above: a wide range of devices have been employed to observe insect behaviour in the laboratory. A
simple arena, consisting of a petri dish may have odour sources, for example, place din the centre. A
perspex box could be used to study the preference of flies to land on certain objects placed within it.
Olfactometers are used to measure the response of an insect to an odour by giving it a choice of
odours. Typically several inlets carry streams of air into the chamber and one or more of these streams
may contain an introduced odour and the insect is then free to move towards or away from the odour or
to behave indifferently to it. A Y-tube choice-chamber provides a convenient set-up in many cases,
with two air streams entering the chamber through guaze meshes (which screen out visual cues and
prevent the insect from escaping. Care is always needed in these experiments. Some insects leave
chemical trails that can be detected by their fellows and this may bias a Y-tube choice experiment, for
example, if the chamber is not cleaned or replaced between trials and insects prefer to follow the paths
of the previous test subject. Insects also have memory, using the same individual repeatedly can bias
results, for example if an insect turned left in a Y-tube and this led to its release from the apparatus,
then it might remember that left leads to escape and prefer to keep turning left on subsequent trials.
Insects make good subjects for the study of animal behaviour. Their nervous systems and behaviours
may be complex, but it is possible to discern mechanisms and rules governing insect behaviour. They
are also generally readily available in large numbers and easy to manipulate in the laboratory.
In this article, we are going to look at the petri-dish arena. The floor of the arena will be covered with
filter paper, which the insects can easily grip and walk across and which can be replaced for each trial,
eliminating any trails that may be deposited. The insects will be filmed under even infra-red illumination,
which most insects can not see, thus eliminating responses to remote light sources, but which we can
detect with an infra-red camera, enabling us to film and record the insects' behaviour. A small drop of
odourant can be added to the centre of the arena, onto the filter paper (or a separate filter-paper disc)
and then an insect added to the arena some distance from the source and its behaviour recorded.
The patterns of movement obtained from such an experiment may be hard to analyses, compared to
say a simpler Y-tube choice experiment. Parameters like how long the insect spends near the source, or
how many times it walks towards it can be measured. However, with computer modeling more complex
analysis is possible.
First of all, we need a control. We should observe a number of insects in the arena in the absence of an
odour source (and also in the presence of just the solvent used to dissolve the odour source, such as
water or paraffin oil). Typically, in odourless controls the following pattern of movement is seen:
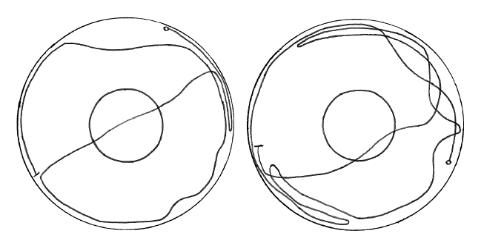
These are two actual recordings of a beetle moving in the arena. Each trace covers 30s of activity. The
outer circle is the edge of the 9 cm diameter petri-dish and the inner circle (3 cm diameter) is the zone
within which an odour would be applied if present. The start of each trace is indicated by a small circle
and the end by a short perpendicular line. This response is typical - the insect tends to avoid the centre
of the arena and in fact prefers to stay near the edge, where it is touching the edge of the petri-dish.
This is positive thigmotaxis, a movement in response to touch in which an insect positions itself in a
place which causes maximum tactile stimulation to its body. In this way insects are drawn to nooks and
crannies where they are generally safer rather than being in the open.
Below are some recording (again each trace covers 30 seconds) in which an odour source associated
with food was placed in the centre of the arena (this is a drop of liquid plant extract):
outer circle is the edge of the 9 cm diameter petri-dish and the inner circle (3 cm diameter) is the zone
within which an odour would be applied if present. The start of each trace is indicated by a small circle
and the end by a short perpendicular line. This response is typical - the insect tends to avoid the centre
of the arena and in fact prefers to stay near the edge, where it is touching the edge of the petri-dish.
This is positive thigmotaxis, a movement in response to touch in which an insect positions itself in a
place which causes maximum tactile stimulation to its body. In this way insects are drawn to nooks and
crannies where they are generally safer rather than being in the open.
Below are some recording (again each trace covers 30 seconds) in which an odour source associated
with food was placed in the centre of the arena (this is a drop of liquid plant extract):
Again the edge of the arena and the 3 cm central zone are indicated, as is a smaller inner circle
(about 1 cm diameter) showing the extent of spread of the plant extract in the filter paper. Using a
plant extract in this way eliminates visual cues, especially with the insect in darkness (infra-red light).
There may be a tactile and/or taste component when the insect encounters the liquid (which has
soaked into the filter paper) and indeed we can see it occasionally stop on the edge of the source
and change direction or reverse. The solvent in this case was water and interestingly with water
alone, one sees either indifference or an attraction towards it which results in the insect stopping and
sitting over the wet patch. Clearly what we see above is not a response to the water solvent, but a
response to the odour of the plant. However, it is not clear what kind of response it is, the insect
tends to walk in tight circles, sometimes centred on the odour source, sometimes off-centre. Is it
attracted to the source? Is it irritated by the source? Is it searching for something associated with the
odour?
Computer models have helped explain this behaviour pattern. The model uses the same software (a
C# program with a Winform interface) that was used to model chemokinesis in bacteria, but with a
different set of parameters. Bacteria respond to attractive or repellent chemicals by altering their
turning frequency and by sampling the chemical concentration at different time points - they
remember what concentration they were exposed to over several seconds, allowing them to
determine whether they are moving away or towards the source. Bacteria are too small to sample a
chemical gradient spatially, since their bodies are too small. An insect, however, can sample the
odour with its antennae, and the antenna nearest the source may receive a stronger stimulus than
the antenna further from it - it measures chemical gradients in space at the same time-point. It can
then simply turn toward the antenna that is more strongly stimulated, if it is attracted to the odour, or
turn away from it if it repelled. This spatial sampling of an odour source and turning towards it is
chemotaxis (though in recent times the distinction in definition between chemotaxis and
chemokinesis is often ignored).
If the insect is attracted to the odour it will undergo positive chemotaxis and we might expect the
insect to move quite accurately towards the source, and in some cases it does. How though do we
explain the curious turning behaviour described above in terms of chemotaxis? A computer model
enables us to alter the strength of the turning response and see what happens.
The result of a computer simulation is shown below (these are actual images of traces generated by
the program). This is a stochastic model - it determines the behaviour of the insect at given time
intervals according to probabilistic rules and parameters, such as what is the probability of turning
left or right and by how many degrees?
(about 1 cm diameter) showing the extent of spread of the plant extract in the filter paper. Using a
plant extract in this way eliminates visual cues, especially with the insect in darkness (infra-red light).
There may be a tactile and/or taste component when the insect encounters the liquid (which has
soaked into the filter paper) and indeed we can see it occasionally stop on the edge of the source
and change direction or reverse. The solvent in this case was water and interestingly with water
alone, one sees either indifference or an attraction towards it which results in the insect stopping and
sitting over the wet patch. Clearly what we see above is not a response to the water solvent, but a
response to the odour of the plant. However, it is not clear what kind of response it is, the insect
tends to walk in tight circles, sometimes centred on the odour source, sometimes off-centre. Is it
attracted to the source? Is it irritated by the source? Is it searching for something associated with the
odour?
Computer models have helped explain this behaviour pattern. The model uses the same software (a
C# program with a Winform interface) that was used to model chemokinesis in bacteria, but with a
different set of parameters. Bacteria respond to attractive or repellent chemicals by altering their
turning frequency and by sampling the chemical concentration at different time points - they
remember what concentration they were exposed to over several seconds, allowing them to
determine whether they are moving away or towards the source. Bacteria are too small to sample a
chemical gradient spatially, since their bodies are too small. An insect, however, can sample the
odour with its antennae, and the antenna nearest the source may receive a stronger stimulus than
the antenna further from it - it measures chemical gradients in space at the same time-point. It can
then simply turn toward the antenna that is more strongly stimulated, if it is attracted to the odour, or
turn away from it if it repelled. This spatial sampling of an odour source and turning towards it is
chemotaxis (though in recent times the distinction in definition between chemotaxis and
chemokinesis is often ignored).
If the insect is attracted to the odour it will undergo positive chemotaxis and we might expect the
insect to move quite accurately towards the source, and in some cases it does. How though do we
explain the curious turning behaviour described above in terms of chemotaxis? A computer model
enables us to alter the strength of the turning response and see what happens.
The result of a computer simulation is shown below (these are actual images of traces generated by
the program). This is a stochastic model - it determines the behaviour of the insect at given time
intervals according to probabilistic rules and parameters, such as what is the probability of turning
left or right and by how many degrees?

In (A) there is no odour source and the computer uses a simple turning algorithm to predict the
behaviour of the insect when it encounters the outer wall of the arena. This accurately predicts the sort
of response we saw in the absence of an odour source. In B to E an attractive chemical is placed at
the centre of the arena and modeled to diffuse out from the centre with concentration diminishing
exponentially from the centre and steady over time. A fixed turn angle of 25 degrees has been used
and a turn probability (per second) of 0.25 in the absence of odour (A) and 0.85 and a slightly variable
speed of about 20-30 mm/s with the turning probability biased towards the source. This produced
circling behaviour similar to our experiment. The insect appears to be responding by turning toward
the source (positive chemotaxis) and by turning with higher frequency (chemokinesis) in the
presence of the odour, but with a turn angle and turn rate that results in the circular motion, rather
than a movement direct toward the source (which can be reproduced by the model with other
parameters). Also note that the circles are not always centred on the source in the computer model.
Other patterns of behaviour were found in response to different odours, such as the response to a
drop of gland extract from another beetle of the same species (from the tergal gland, thought to be
used for communication in some beetles):
behaviour of the insect when it encounters the outer wall of the arena. This accurately predicts the sort
of response we saw in the absence of an odour source. In B to E an attractive chemical is placed at
the centre of the arena and modeled to diffuse out from the centre with concentration diminishing
exponentially from the centre and steady over time. A fixed turn angle of 25 degrees has been used
and a turn probability (per second) of 0.25 in the absence of odour (A) and 0.85 and a slightly variable
speed of about 20-30 mm/s with the turning probability biased towards the source. This produced
circling behaviour similar to our experiment. The insect appears to be responding by turning toward
the source (positive chemotaxis) and by turning with higher frequency (chemokinesis) in the
presence of the odour, but with a turn angle and turn rate that results in the circular motion, rather
than a movement direct toward the source (which can be reproduced by the model with other
parameters). Also note that the circles are not always centred on the source in the computer model.
Other patterns of behaviour were found in response to different odours, such as the response to a
drop of gland extract from another beetle of the same species (from the tergal gland, thought to be
used for communication in some beetles):

It would be interesting to see what parameters of turning rate and turning angle recreate these
patterns in our computer model. In conclusion, prior to developing the computer model it was very
difficult indeed to make sense of the behaviours observed by experiment.
The Y-tube Olfactometer
The Y-tube olfactometer has also been used to ascertain odour preferences in Aleochara bilineata.
This rove beetle is a predator and parasitoid of certain root-fly maggots, such as cabbage-root fly
(Delia radicum) and onion-root fly (Delia antiqua). [See the life-cycle of Aleochara bilineata.] The adult
female first locates the host plant, such as a cabbge plant, e.g. rutabaga (swede), preferably one that
is already infested with root flies and then lays her eggs in the soil near the plant. The hatchlings ahve
the task of locating a host pupa. The fly maggots typically crawl away from the plant roots some
distance into the soil in order to pupate, and the hatchlings burrow after them. Note that males may
also be attracted to the plants in order to find females, and there is evidence that a number of adult
beetles set-up communal burrows in the soil around the infested plant. The adult's have been tested
by scientists in a Y-tube olfactometer. Typically, air is drawn from the two arms of the Y-tube down the
stem and out, so that an insect introduced into the stem of the Y-tube encounters air streams from two
sources, the left and right arms. This allows comparisons to be made, for example the left arm might
blow in fresh air, while the right arm may blow in air passed over a piece of rutabaga. When the insect
arrives at the fork it can then make an informed choice as to whether to turn left or right. If it is
attracted to the rutabaga then it will turn right more often than left, over a number of trials. This would
be repeated with say 50 different insects (or ten insects each tested 5 times) to give a statistically
large enough sample to see whether or not the insects prefer to turn toward the rutabaga.
First of all it is clear that the beetles learn from this experience. When an insect is introduced into the
stem of the Y-tube and crawls forward, it will turn left or right and then be released. It has been shown
that some insects, including Aleochara bilineata, can remember whether they turned left or right on
their first time in the olfactometer, for example supposing a beetle has been placed in the Y-tube once
and turned left and then was released, it will remember that it escaped by turning left and there is a
statistical tendency for it to turn left on subsequent trials, with the aim to escape! This can be
accounted for, either by using each insect once only (which requires a lot of insects) or by reversing
the odour sources (e.g. have the rutabaga on the right arm for one trial, and then on the left for the
next trial, then right, then left, etc.). There may also be problems with insects leaving odour trails in the
Y-tube that others may follow, in which case it may require a thorough clean between each trial. The
sources should also be masked, say by a piece of clothe over the two ends of the Y-tube, so that they
can not see that an object (a piece of rutabaga) is on the right and head for cover! The size of the
Y-tube will also impact on the results and the optimum size may be different for different insect
species. Even the weather may have an effect, for example research has shown that if whiteflies are
being tested in a Y-tube they tend not to move at all if the weather outside the lab is stormy! All in all,
these experiments can be difficult and time-consuming, and require a lot of patience!
Nevertheless, one group has obtained results for Aleochara bilineata. They found that it is attracted to
rutabaga and even more attracted to rutabaga infected by cabbage-fly maggots. They also found that
they are strongly attracted to the smell of larval frass (faeces) and to the maggots themselves, unless
the maggots were washed clean beforehand!
Other studies have also shown that Aleochara bilineata is attracted to certain chemicals that
characterise the host plant, such as dimethyl sulphide, which is produced by onions.
Electroantennograms
This is an electrophysiological method that is often used to supplement behavioural experiments. It
involves attaching electrodes to the antenna and the head of an insect and then measuring the
electric voltage generated when the antenna is stimulated by various odours. The electrodes will
measure the whole-organ electric potential generated by nerve cells in the antenna when these
become activated by an odour the antenna is sensitive to. The larger the voltage, the more sensitive
the antenna is taken to be to that odour (although the brain may modify this sensitivity and so we can
say nothing about what the insect actually perceives, only what the antenna detects). These studies
have been carried out on Aleochara bilineata, though I don't currently have access to the data and will
report on these studies when i have seen the data.
Control Systems
A totally different approach to the study of insect behaviour looks at control systems. Such studies
have been carried out on other animals too, but insects make ideal subjects for study, as you will see.
Cybernetics is the study of control systems - how a system, such as an animal or one of its
sub-systems, regulates its internal and external environments. The study of animal control systems,
important in its own right, is also proving crucial to the advancement of robotics. Engineers find that a
robot needs control systems to make sure it does what it is supposed to do, such as walk without
falling over or bumping into things. I feel that further research into animal control systems would help
advance robotics further, and insects make ideal subjects for study. As has been said by others,
insects are perfect little robots!
For example, insect antennae contain a sensor called Johnston's organ, which measures deflection of
the antenna. This is used, for example, to measure wind speed in a flying insect: as the insect flies
through the air, the oncoming air stream bends back the antennae and Johnston's organ measures
this deflection. If the insect flies faster or is heading into a stronger headwind then deflection of the
antennae increases. An insect can be tethered in the lab, suspended from some device, perhaps a
force transducer, attached to its back. If air is then blown into its face it will begin to flap its wings,
perhaps fooled into thinking that is flying anyway. If the wind increases then it will reduce its speed and
actively bend its antennae forwards, maintaining a constant deflection of the antennae and
maintaining its flight speed. (If an untethered insect were to speed up then the wind speed would
increase, so wind speed is a measure of flight speed). How does the insect do this? Clearly Johnston's
organ sends signals to the brain whenever wind speed or flights peed changes, as some kind of
feedback.
An example of relevance to most animals, including humans and insects, is motion of the eyes. When
your head moves, how do you know that your head is moving and not your whole body or the outside
world whizzing past? A classic example that has been studied is prey-capture in the preying mantis. If
you ever tried to catch a fly then you will know how hard it can be using speed alone. However, the
preying mantice is an expert fly-catcher! It has good vision to detect the fly and then very rapidly
strikes with its forelegs whilst lunging forwards with its mid and hindlegs. The forelegs have special
spines on the femur, which trap the fly against the tibia, a feat that requires exquisite precision at high
speeds. How do they do this? The explanation is technical, but I shall explain it carefully, beginning
with some basic principles of control systems.
There are three main types of control system that we shall consider. We shall begin with the abstract
principles, talking about subsystems, inputs and outputs. These could refer to anything in an animal or
machine that takes sensory input and produces some output, that is anything which processes signals,
and we shall give insect examples later. These three types are:
1) The chain
In a chain the subsystems are connected in series. We shall consider only two subsystems, A and B.
The input to the chain is input into the first subsystem, A, then the output from A is input into B and
then B generates the output of the chain:
patterns in our computer model. In conclusion, prior to developing the computer model it was very
difficult indeed to make sense of the behaviours observed by experiment.
The Y-tube Olfactometer
The Y-tube olfactometer has also been used to ascertain odour preferences in Aleochara bilineata.
This rove beetle is a predator and parasitoid of certain root-fly maggots, such as cabbage-root fly
(Delia radicum) and onion-root fly (Delia antiqua). [See the life-cycle of Aleochara bilineata.] The adult
female first locates the host plant, such as a cabbge plant, e.g. rutabaga (swede), preferably one that
is already infested with root flies and then lays her eggs in the soil near the plant. The hatchlings ahve
the task of locating a host pupa. The fly maggots typically crawl away from the plant roots some
distance into the soil in order to pupate, and the hatchlings burrow after them. Note that males may
also be attracted to the plants in order to find females, and there is evidence that a number of adult
beetles set-up communal burrows in the soil around the infested plant. The adult's have been tested
by scientists in a Y-tube olfactometer. Typically, air is drawn from the two arms of the Y-tube down the
stem and out, so that an insect introduced into the stem of the Y-tube encounters air streams from two
sources, the left and right arms. This allows comparisons to be made, for example the left arm might
blow in fresh air, while the right arm may blow in air passed over a piece of rutabaga. When the insect
arrives at the fork it can then make an informed choice as to whether to turn left or right. If it is
attracted to the rutabaga then it will turn right more often than left, over a number of trials. This would
be repeated with say 50 different insects (or ten insects each tested 5 times) to give a statistically
large enough sample to see whether or not the insects prefer to turn toward the rutabaga.
First of all it is clear that the beetles learn from this experience. When an insect is introduced into the
stem of the Y-tube and crawls forward, it will turn left or right and then be released. It has been shown
that some insects, including Aleochara bilineata, can remember whether they turned left or right on
their first time in the olfactometer, for example supposing a beetle has been placed in the Y-tube once
and turned left and then was released, it will remember that it escaped by turning left and there is a
statistical tendency for it to turn left on subsequent trials, with the aim to escape! This can be
accounted for, either by using each insect once only (which requires a lot of insects) or by reversing
the odour sources (e.g. have the rutabaga on the right arm for one trial, and then on the left for the
next trial, then right, then left, etc.). There may also be problems with insects leaving odour trails in the
Y-tube that others may follow, in which case it may require a thorough clean between each trial. The
sources should also be masked, say by a piece of clothe over the two ends of the Y-tube, so that they
can not see that an object (a piece of rutabaga) is on the right and head for cover! The size of the
Y-tube will also impact on the results and the optimum size may be different for different insect
species. Even the weather may have an effect, for example research has shown that if whiteflies are
being tested in a Y-tube they tend not to move at all if the weather outside the lab is stormy! All in all,
these experiments can be difficult and time-consuming, and require a lot of patience!
Nevertheless, one group has obtained results for Aleochara bilineata. They found that it is attracted to
rutabaga and even more attracted to rutabaga infected by cabbage-fly maggots. They also found that
they are strongly attracted to the smell of larval frass (faeces) and to the maggots themselves, unless
the maggots were washed clean beforehand!
Other studies have also shown that Aleochara bilineata is attracted to certain chemicals that
characterise the host plant, such as dimethyl sulphide, which is produced by onions.
Electroantennograms
This is an electrophysiological method that is often used to supplement behavioural experiments. It
involves attaching electrodes to the antenna and the head of an insect and then measuring the
electric voltage generated when the antenna is stimulated by various odours. The electrodes will
measure the whole-organ electric potential generated by nerve cells in the antenna when these
become activated by an odour the antenna is sensitive to. The larger the voltage, the more sensitive
the antenna is taken to be to that odour (although the brain may modify this sensitivity and so we can
say nothing about what the insect actually perceives, only what the antenna detects). These studies
have been carried out on Aleochara bilineata, though I don't currently have access to the data and will
report on these studies when i have seen the data.
Control Systems
A totally different approach to the study of insect behaviour looks at control systems. Such studies
have been carried out on other animals too, but insects make ideal subjects for study, as you will see.
Cybernetics is the study of control systems - how a system, such as an animal or one of its
sub-systems, regulates its internal and external environments. The study of animal control systems,
important in its own right, is also proving crucial to the advancement of robotics. Engineers find that a
robot needs control systems to make sure it does what it is supposed to do, such as walk without
falling over or bumping into things. I feel that further research into animal control systems would help
advance robotics further, and insects make ideal subjects for study. As has been said by others,
insects are perfect little robots!
For example, insect antennae contain a sensor called Johnston's organ, which measures deflection of
the antenna. This is used, for example, to measure wind speed in a flying insect: as the insect flies
through the air, the oncoming air stream bends back the antennae and Johnston's organ measures
this deflection. If the insect flies faster or is heading into a stronger headwind then deflection of the
antennae increases. An insect can be tethered in the lab, suspended from some device, perhaps a
force transducer, attached to its back. If air is then blown into its face it will begin to flap its wings,
perhaps fooled into thinking that is flying anyway. If the wind increases then it will reduce its speed and
actively bend its antennae forwards, maintaining a constant deflection of the antennae and
maintaining its flight speed. (If an untethered insect were to speed up then the wind speed would
increase, so wind speed is a measure of flight speed). How does the insect do this? Clearly Johnston's
organ sends signals to the brain whenever wind speed or flights peed changes, as some kind of
feedback.
An example of relevance to most animals, including humans and insects, is motion of the eyes. When
your head moves, how do you know that your head is moving and not your whole body or the outside
world whizzing past? A classic example that has been studied is prey-capture in the preying mantis. If
you ever tried to catch a fly then you will know how hard it can be using speed alone. However, the
preying mantice is an expert fly-catcher! It has good vision to detect the fly and then very rapidly
strikes with its forelegs whilst lunging forwards with its mid and hindlegs. The forelegs have special
spines on the femur, which trap the fly against the tibia, a feat that requires exquisite precision at high
speeds. How do they do this? The explanation is technical, but I shall explain it carefully, beginning
with some basic principles of control systems.
There are three main types of control system that we shall consider. We shall begin with the abstract
principles, talking about subsystems, inputs and outputs. These could refer to anything in an animal or
machine that takes sensory input and produces some output, that is anything which processes signals,
and we shall give insect examples later. These three types are:
1) The chain
In a chain the subsystems are connected in series. We shall consider only two subsystems, A and B.
The input to the chain is input into the first subsystem, A, then the output from A is input into B and
then B generates the output of the chain:
2) The mesh
In this case, using two subsystems A and B, the inputs of both subsystems, A and B, are added or
subtracted (by an accumulator) to produce the output of the system:
In this case, using two subsystems A and B, the inputs of both subsystems, A and B, are added or
subtracted (by an accumulator) to produce the output of the system:

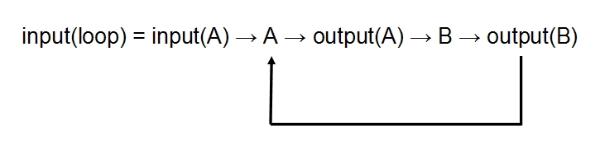

3) The Loop
A and B are chained as in (1) but the output from B joins the input of A, forming a closed loop:
A and B are chained as in (1) but the output from B joins the input of A, forming a closed loop:
Both (1) and (2) are open-control systems, in that at no point is the final output fed back into the system.
The loop (3) is a closed-control system, since the output is fed back into the system as input. This is a
feedback loop.
Examples:
An example of a chain is given by the firefly Photinus. Males locate females by the flashes of light they
emit. When males are exposed to a brief flash of light, they will turn to face the light, even if the duration of
the flash is less than their reaction time (meaning there can be no corrective feedback to alter their
position). The flash of light inputs on the visual subsystem which sends output to the effector subsytem
which turns the insect:
The loop (3) is a closed-control system, since the output is fed back into the system as input. This is a
feedback loop.
Examples:
An example of a chain is given by the firefly Photinus. Males locate females by the flashes of light they
emit. When males are exposed to a brief flash of light, they will turn to face the light, even if the duration of
the flash is less than their reaction time (meaning there can be no corrective feedback to alter their
position). The flash of light inputs on the visual subsystem which sends output to the effector subsytem
which turns the insect:

An example of a feedback loop (3) is found in the optomotor reflex of the hoverfly Eristalis. If placed
inside a vertical cylinder with vertical stripes painted on it, the fly will track the stripes, so that if the drum or
cylinder is rotated, the fly will turn so as to track it and maintain its position with respects to the drum. This
tracking is the optomotor reflex. For example, if the drum is rotated clockwise then the fly will turn
clockwise, maintaining its relative position to the stripes, this is the example of a closed feedback loop.
Such a response does not occur when the fly moves of its own accord: it can distinguish changes in
sensory inputs due to its own behaviour from that due to external changes in the environment. A change
in the position of the stripes is detected by the compound eyes and this visual subsystem then sends its
output to the effector subsystem causing the fly to turn and the visual system then feeds back the change
in position resulting from the fly turning. In other words the output of the system, the fly turning, is fed-back
into the system as altered visual input to the visual subsystem.
inside a vertical cylinder with vertical stripes painted on it, the fly will track the stripes, so that if the drum or
cylinder is rotated, the fly will turn so as to track it and maintain its position with respects to the drum. This
tracking is the optomotor reflex. For example, if the drum is rotated clockwise then the fly will turn
clockwise, maintaining its relative position to the stripes, this is the example of a closed feedback loop.
Such a response does not occur when the fly moves of its own accord: it can distinguish changes in
sensory inputs due to its own behaviour from that due to external changes in the environment. A change
in the position of the stripes is detected by the compound eyes and this visual subsystem then sends its
output to the effector subsystem causing the fly to turn and the visual system then feeds back the change
in position resulting from the fly turning. In other words the output of the system, the fly turning, is fed-back
into the system as altered visual input to the visual subsystem.
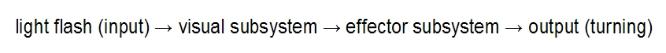
Negative and positive feedback. The feedback is normally negative, in that once the fly changes position
cut-through and then the head turned 180 degrees and fastens in place to the thorax (!) the fly turns in
the wrong direction, increasing the discrepancy between stimulus and desired result, so that it turns faster
and faster (turning anticlockwise if the cylinder rotates clockwise) until it fall over. The feedback has
become positive!
Control systems of prey capture in mantids have been studied by Mittelstaedt (1962) and Corrette (1990)
who expanded on and corrected Mittelsteadt's pioneering work. For prey capture the mantid wants to align
its head and body with the target fly, more-or-less, bringing the attack region which is about 20 degrees to
either side. That is it strikes approximately straight forward, so we it aims the head and body rather than
firing its legs off to one-side. It wants the angles x and y, in the diagram below, to be as close as possible
to zero, before striking. The head is joined by the cervix (neck) to the first thoracic segment, the prothorax.
On the front of the prothorax are two pairs of sensory hair plates. These are proprioceptors, sensors
that measure the relative movement of body parts. If the head is turned to one side, then it brushes
against the hair plates, deflecting the bristles or hairs, triggering nerve impulses to be sent to the brain.
The more the head turns, the more hairs that become deflected and the more strongly they deflect and
the stronger the nerve impulses. Thus, the mantid can measure the position of its head relative to its body.
The pair of compound eyes of the mantid have two forward areas where the vision is especially sharp,
called foveas. When the foveas focus on the target the difference in stimulus of each eye allows the
distance to the target to be measured by binocular vision. The range of the strike is about 25 mm in
front of the head.
cut-through and then the head turned 180 degrees and fastens in place to the thorax (!) the fly turns in
the wrong direction, increasing the discrepancy between stimulus and desired result, so that it turns faster
and faster (turning anticlockwise if the cylinder rotates clockwise) until it fall over. The feedback has
become positive!
Control systems of prey capture in mantids have been studied by Mittelstaedt (1962) and Corrette (1990)
who expanded on and corrected Mittelsteadt's pioneering work. For prey capture the mantid wants to align
its head and body with the target fly, more-or-less, bringing the attack region which is about 20 degrees to
either side. That is it strikes approximately straight forward, so we it aims the head and body rather than
firing its legs off to one-side. It wants the angles x and y, in the diagram below, to be as close as possible
to zero, before striking. The head is joined by the cervix (neck) to the first thoracic segment, the prothorax.
On the front of the prothorax are two pairs of sensory hair plates. These are proprioceptors, sensors
that measure the relative movement of body parts. If the head is turned to one side, then it brushes
against the hair plates, deflecting the bristles or hairs, triggering nerve impulses to be sent to the brain.
The more the head turns, the more hairs that become deflected and the more strongly they deflect and
the stronger the nerve impulses. Thus, the mantid can measure the position of its head relative to its body.
The pair of compound eyes of the mantid have two forward areas where the vision is especially sharp,
called foveas. When the foveas focus on the target the difference in stimulus of each eye allows the
distance to the target to be measured by binocular vision. The range of the strike is about 25 mm in
front of the head.
1) The proprioceptive input (y) is added to the visual input (x) and the mantid will strike when the sum is
close enough to zero. This is a mesh, let's call it a proprioceptive mesh.
2) When the brain sends an order to move the head, it also sends a copy of what the expected result
should be, called the efferent copy, to a region of the nervous system called the comparator. The
efferent copy is what the eyes should see if the head only has moved and not the external objects in the
visual field themselves. The visual subsystem also sends its outputs to the comparator, which subtracts the
new visual subsystem output from the efferent copy. If the head only has moved then the comparator
output should be zero, otherwise something moved other than the insect. We can call this model a copy
mesh.
3) We can have a feedback loop in which the optic output from the visual subsystem acts on the input to
the efferent subsystem by negative feedback. This is similar to our Eristalis in the rotating cylinder, the
mantid will turn its head towards the target - it will know which way to turn according to whether the left or
right compound eye is more greatly stimulated - and then the new optic output will feed to the effector
subsystem telling it to turn the head left or right by a lesser amount until the target is at the center of the
visual field when the head stops turning (we assume a stationary target for simplicity). This is the fixed
reference point, x = 0, which the system aims to achieve. We call this control system the optic loop. Loops
fall into two main types: those with fixed reference points, such as the optic loop, and those with variable
reference points.
Experiments seem to have ruled out the first model, the proprioceptive mesh. If the mantid head is turned
10 to 20 degrees to the left and fastened to the prothorax in its new position then the mantid misses
70-80% of strikes to the right and continues like this for days without learning to compensate. This
suggests that the proprioceptive output is not feeding into the input of the visual system, as in a loop, but
that the system is open (a chain or mesh). Mittelstaedt (1962) considered that these experiments ruled out
the proprioceptive mesh also. He made one false assumption, however, that the mantids calculated the
angle of the thorax to the prey in order to strike at the right angle, but in actual fact they tend rather to
align the thorax and head with the target before striking when allowed to move freely. In the former scheme
the proprioceptive mesh would have to add angles x + y to obtain the strike angle z. In the latter scheme x
and y are adjusted to equal approximately zero. In the former scheme altering the head angle would simply
fix angle y and then the strike angle could easily change to compensate, but as it didn't this ruled out the
proprioceptive mesh model. With the correction to their behaviour applied it is clear that they would not be
able to compensate as they can not make both x and y equal zero.
Cutting the sensory nerves from the proprioceptive plates on the left side (deafferentation) caused
mantids to nearly always miss prey by striking to the right of the target. This seemed to favour the
proprioceptive mesh, however deafferentation also affected the neck muscles and these mantids have
their heads cocked to the left. Thus they were overestimating the angle y and over-compensated by
striking to the right. After 14-20 days both the missing bias and the head tilting bias disappeared as the
mantids compensated. It also suggests that the neck muscles and the neck proprioceptors operate in a
feedback loop, so that feedback from the proprioceptors maintains tone in the neck muscles and hence
head position, maintaining the desired angle of the head to the thorax (y):
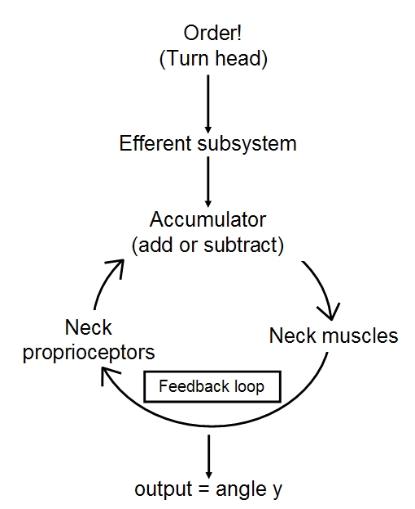
point of x = 0, this loop has a changing reference point, the desired angle, y, of the head. In
prey-capture this loop may serve to center the head on the target.
Having established that the optic and efferent subsystems both utilise feedback loops, what is less
obvious is how these two subsystems interact. Mittelstaedt favoured our second model - the copy mesh. In
the outflow theory, developed by Helmholtz (1867) when the brain (could be a human brain) sends
instructions to the eye muscles to move the eye, it also sends an efferent copy to the comparator which
compares this expected result with the output of the visual system by subtracting one from the other. If,
after the eye movement, the result of this comparison is zero, then the visual field is as expected and so
any changes are due entirely to the eye movement. If it is not zero, then any difference is due to changes
in the external objects being observed (or an external force moving the head or body) and motion is
perceived. This is our copy comparator. According to the inflow theory, developed by Sherrington
(1918) proprioceptors in the eye muscles send sensory output to the comparator. The eye muscles do
indeed contain proprioceptors, however, simply deforming the eyeball with one's finger causes movement
to be perceived, so signals from the proprioceptors do not appear to be compared in the comparator in
this case. Other studies suggest that the eye muscle proprioceptors are not used by the visual system in
sensing eye position, so the outflow theory is favoured.
The Reafference Principle
In 1950, Holst and Mittelstaedt developed the reafference principle. This states that the comparator
compares exafferent information, that is changes in the visual field not accounted for by body
movements and due to external changes, with reafferent information, that is changes in the visual field
expected to result from body movements. If the reafferent and exafferent inputs cancel then sensed
movement is due entirely to changes in eye position (eye or body movement). This principle can be
applied to any sense organ that senses external stimuli and is affected by body movements, not just vision.
Such a reafference system is illustrated below:
prey-capture this loop may serve to center the head on the target.
Having established that the optic and efferent subsystems both utilise feedback loops, what is less
obvious is how these two subsystems interact. Mittelstaedt favoured our second model - the copy mesh. In
the outflow theory, developed by Helmholtz (1867) when the brain (could be a human brain) sends
instructions to the eye muscles to move the eye, it also sends an efferent copy to the comparator which
compares this expected result with the output of the visual system by subtracting one from the other. If,
after the eye movement, the result of this comparison is zero, then the visual field is as expected and so
any changes are due entirely to the eye movement. If it is not zero, then any difference is due to changes
in the external objects being observed (or an external force moving the head or body) and motion is
perceived. This is our copy comparator. According to the inflow theory, developed by Sherrington
(1918) proprioceptors in the eye muscles send sensory output to the comparator. The eye muscles do
indeed contain proprioceptors, however, simply deforming the eyeball with one's finger causes movement
to be perceived, so signals from the proprioceptors do not appear to be compared in the comparator in
this case. Other studies suggest that the eye muscle proprioceptors are not used by the visual system in
sensing eye position, so the outflow theory is favoured.
The Reafference Principle
In 1950, Holst and Mittelstaedt developed the reafference principle. This states that the comparator
compares exafferent information, that is changes in the visual field not accounted for by body
movements and due to external changes, with reafferent information, that is changes in the visual field
expected to result from body movements. If the reafferent and exafferent inputs cancel then sensed
movement is due entirely to changes in eye position (eye or body movement). This principle can be
applied to any sense organ that senses external stimuli and is affected by body movements, not just vision.
Such a reafference system is illustrated below:

References / further reading
Mittelsteadt, H. 1962. Control systems of orientation in insects. Ann. Rev. Entomol. 7:177-198.
Corrette, B. J. 1990. Prey capture in the praying mantis Tenodera aridifolia sinensis: coordination of the
capture sequence and strike movements. J. exp. Biol. 148: 147-180.
McFarland, D. 1985. Animal Behaviour: Psychology, Ethology and Evolution. Longman Scientific &
Technical (Pub.).
Article amended: 28 Dec 2015

