| Jelly
Creatures - Siphonophores |
Physalia, the
Portuguese Man-of-War
(Portuguese Man o' War or bluebottle), is found in all oceans,
but prefers the warmer waters of the Indian and Pacific oceans.
It has a remarkable float, usually bluish (sometimes violet) in
colour and typically up to 30 cm long or more. The float and its
crest or sail can be seen above the water with the long
tentacles trailing beneath the water's surface. Two species are
actually recognised, Physalis
physalis
or Portuguese Man o' War and the smaller Physalis
utriculus
or Pacific Man o' War (though probably both are commonly
referred to as Portuguese Man o' War).
These creatures float on the waters, sailing by means of the
collapsible crest or sail (which seems very variable in size
judging from various photographs) dragging their fishing
tentacles through the water. The tentacles are armed with
stinging nematocysts and the venom of the larger species, Physalia physalis, can be lethal to
humans. The tentacles typically reach up to 10 m to 30 m in
length, though there are reports of tentacles growing up to 50
m.
livid
and seemingly synthetic hue
of burgundy or murex or midnight blue,
it drifts in temperate seas
as in irons, the ruffled float
riffling in the breeze
(First verse of a poem by George Bradley, entitled, 'Its
Bladderlike Sail', Grand Street, Vol. 4, No. 4 (Summer,
1985), pp. 109-110).
External
link: siphonophore photos, including a very good photo of Physalia:
http://www2.hawaii.edu/~cdunn/siphonophores.html
These
creatures belong to the Coelenterates and are commonly referred
to as jellyfish, along with the scyphozoa, or jellyfish proper.
(Zoologists frequently only consider the medusae of the
scyphozoa as 'jellyfish' though the term is commonly used in a
broader sense, and why not? 'Jellyfish' is after all a
descriptive term and not a precise scientific term, much as the
word 'worm' can be applied to a wide range of creatures,
including certain vertebrates). The group of coelenterates to
which Physalis belongs is a remarkable group called the
siphonophores. Like Scyphozoa (e.g. the Moon jellyfish) the
siphonophores originally had a life-cycle involving an
alternation of generations between sessile non-sexual polyps and
free-swimming sexual medusae. However, the life-cycle in
siphonophores is radically altered. Siphonophores grow from
single floating polyps which multiply asexually by budding, but
with the progeny remaining physically and intimately connected
to one-another to form a single composite or colonial organism.
In the Portuguese Man-of-War the float is a single 'individual',
called the pneumatophore, and each
tentacle is an individual - the long fishing tentacles are
called dactylozooids (dactylozoids -
consist of a palpon (feeler) bearing the long tentacle and
sometimes called a tentacular palpon considered unique
to Physalia) and function only to capture food and
defend the colony - being incapable of feeding themselves, as is
the float. Actually, the float was originally regarded as an
individual derived from a modified medusa, but is now known to
be derived from part of the larval individual who founded the
colony.
The
tentacles hang down from half of the float, along with the
shorter feeding polyps called gastrozooids (gastrozoid). Each
gastrozooid has a mouth and a short tentacle and feeds the
colony (some later lose their mouth and develop into long
dactylozooids).
Also
in the colony are short gonozooids (gonozoids) which bear
numerous spheroidal female gonophores and male gonophores - the gonophores are
the sexual 'organs' and are really modified medusae that remain
attached to the colony and are incapable of swimming. Odd
gelatinous zooids may also be present, which resemble simple
gelatinous projections and have an unknown function (may
represent bracts - see below). in Physalia, the gonozooids are
reduced to branched stalks (called gonodendra, singular:
gonodendron) which bear grape-like clusters of gonophores and nectophores
- modified bell-like medusae that can swim - which may propel
the gonodendron when it detaches, as it does when ripe.
Gonopalpons (sensory and/or excretory zooids) and bract-like
jelly polyps (of unknown function) are also borne on the
gonodendra.
The various zooids originated from a single individual and
continue to bud or branch from zooids growing beneath the float,
with the zooids grouped in bunches or modules called cormidia
(singular cormidium). The tentacles cluster on one-side of the
float, either the left side or the right side. Thus, some
individuals have their tentacles on the left and sail on the
right (so-called right-handed individuals) and these sail on the
port tack. Other individuals have their tentacles on the right
and sail on the left (left-handed individuals) and these sail on
the starboard tack (ref. 1). The gastrovascular systems of all
the individuals are interconnected, so that food consumed by the
gastrozooids may be shared by all. (Remember that in
coelenterates the circulatory system is part of the gut or
stomach and forms the gastrovascular cavity).
What we see here is a division of labor - the pneumatophore
gives the colony buoyancy, the dactylozooids catch food and
defend the colony and the gastrozooids ingest food and the
gonozooids function in reproduction.
Some siphonophores have units called dactylozooids (palpons or feelers) which have no mouth and an unbranched basal tentacle or the entire zooid may be modified into a hollow tentacle-like structure. When associated with gonophores, dactylozooids are called gonopalpons.
Rather than floats, some siphonophores have swimming bells or nectophores (nectocalyx) which are modified medusae with a bell and velum (shelf of tissue just inside the bell margin that assists in jet production) 4 radial canals, a ring canal but no manubrium and no mouth, no tentacles and no obvious sense organs. Nectophores are of course muscular.
Bracts
are units that are generally thick, gelatinous and prismatic,
domed or leaf-like (and thus also called phyllozooids).
Each possesses a gastrovascular canal that may be branched.
bracts are of medusoid origin. Bracts have a protective function
as gelatinous shields that conceal the other members when the
members are retracted. In forms lacking nectophores, coordinated
movements of the bracts provide some swimming ability.
The contractile tentacle of each gastrozooid in siphonophores may have lateral branched called tentilla, each of which ends in a knob or coil of nematocysts. The knob bears parallel arched rows of nematocysts (called the cnidoband) and may have a hanging terminal filament also full of nematocysts. For example, Stephanophyes has about 1700 nematocysts per complex, of 4 different types. alternatively, the cnidoband is extended into a spiral coil (which may have a protective cover or involucre over the base) and ends in one or more filaments or in a nematocyst-free sac or ampulla, or in free filaments. The base of the gastrozooid may also be armed with nematocysts. Some siphonophores have 5 or 6 different types of nematocyst.
Siphonophore
gonophores may be medusoid in form with a bell, velum and
radial canals and a manubrium bearing gonads but have no mouth,
no tentacles and no obvious sense organs. they may be released
to shed their gametes before perishing. In some forms, the
gonophores, particular the male ones, are reduced to rounded
sacs. Siphonophores are dioecious, in the sense that an
individual gonophore is of only one sex, but colonies are
usually hermaphroditic: clusters of gonophores may be of
the same sex or mixed. Terms like monoecious and dioecious may
be applied to individual gonophores or to colonies and usage
seems a bit confused. Siphonophores may be protandrous, that is
the male gonophores may mature and ripen first, followed by the
female gonophores, favouring cross-fertilisation.
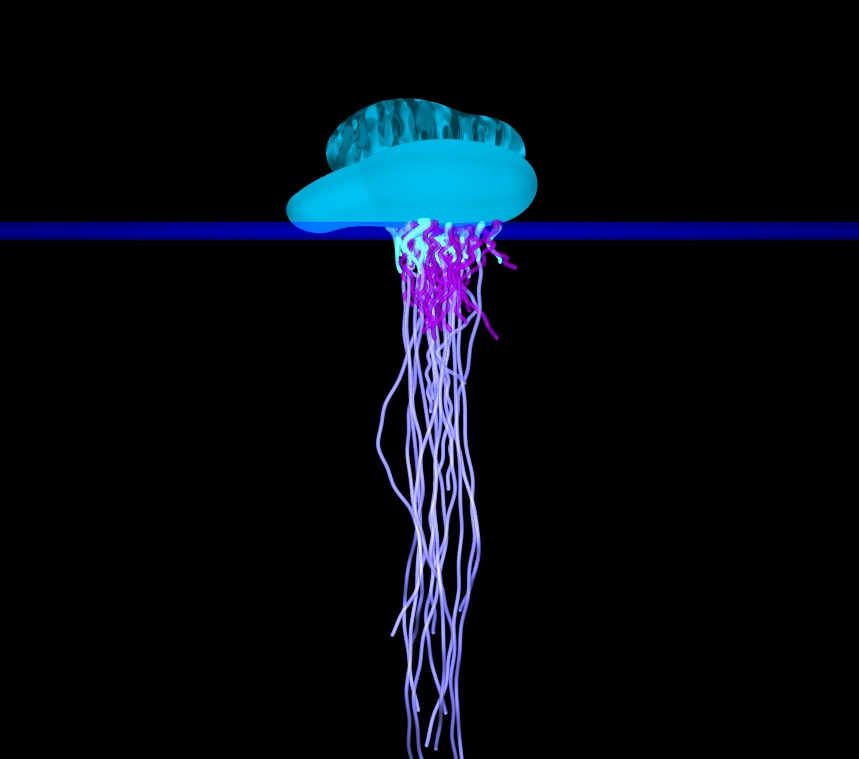
Behavior
The
sail can be collapsed and erected as required and the float
deflated or inflated. The float may deflate, for example, in
stormy weather, whilst the tentacles retract and then spread out
near the surface to increase stability. The float may also
deflate in excess sunshine, to prevent it from drying up. Dipping (2) is a behavior
frequently seen, in which the sail collapses and the float is
turned to one side and dipped in the water to wet it. This
behavior increases with wind speed and in a sense prevents the
float from drying up. However, strictly speaking the floats do
not dry as such, but as water evaporates from the windward side,
salt from the water is deposited on the surface and this salt
will draw out water by osmosis. To prevent this, the sail dips
in order to wash off the salt (indeed, spraying on salty water
triggers the response so the deposition of salt on the
float/sail is the critical signal). The float then rights itself
and the sail re-inflates. The sail has partial chitinous
partitions within it, to give it rigidity when inflated.
The float is usually said to contain a mixture of gases similar
in composition to air, though with less oxygen, however, studies
have shown that a variable amount of the toxic gas carbon
monoxide is also present, sometimes accounting for 35% of the
gas (ref. 3). This carbon monoxide is secreted by a gland (the pneumadena),
comprising a single layer of cells, in the base of the float.
The float is a double-walled chamber. The outer wall is called
the pneumatocodon and the inner wall the pneumatosaccus and in
between the two is the gastrovascular cavity of the float. The
float also possesses muscle fibers to control its
volume and shape and to perform behaviors such as dipping.
Most siphonophores have a different arrangement of polyps. The
polyps bud from a long stalk that hangs in the water (this stalk
is essentially compressed to a disc in Physalia)
and there may or may not be a float and instead swimming medusae,
still attached to the colony, provide propulsion. Some forms have
both a float and swimming medusae. The epidermis of siphonophores,
which connects all the individuals, can conduct electrical signals
(in the absence of nerves) and acts as a neuroid system.
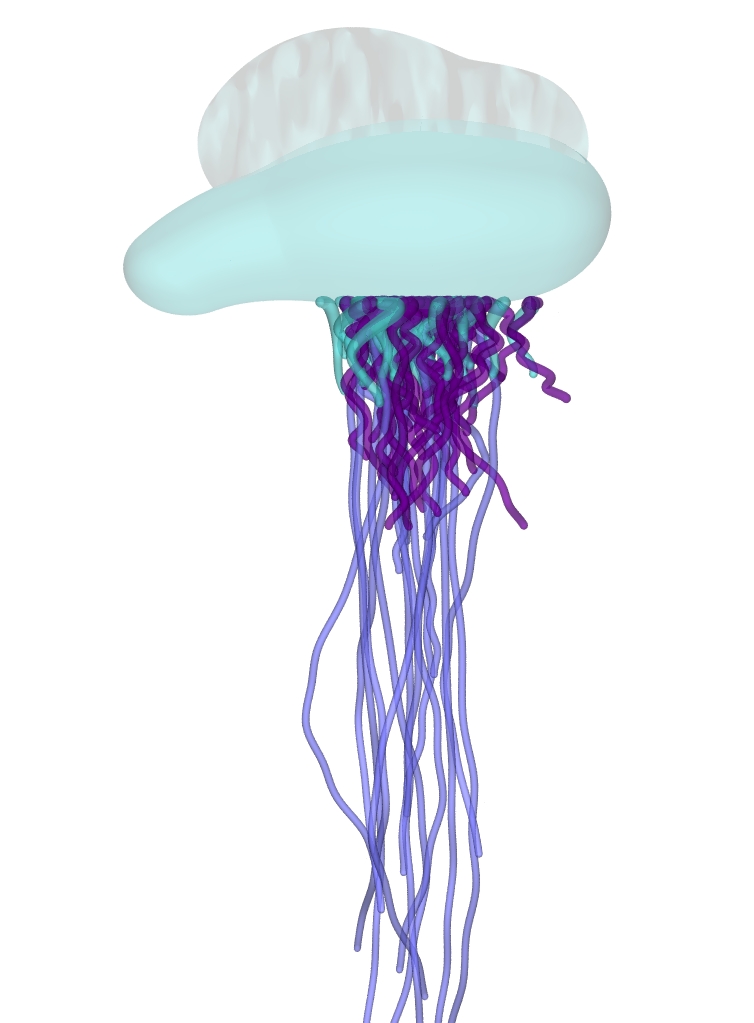
Above:
Physalia floating on the
water's surface.
Physalia has proven difficult to keep in aquaria as it
typically degenerates after a day or two in captivity. It has
been suggested that the long tentacles touching the bottom of
the tank may trigger this degeneration. It is suggested that
tanks should be at least 50 feet deep and have fans positioned
so as to blow the Physalia and keep it in the
center of the tank.
Reproduction
The
gonophores shed their egg and sperm into the sea where
fertilization takes place. The resultant larva develops into a
juvenile colony with a single gastrozoid (protozooid) beneath
and one tentacle. The pneumatophore develops from an
invagination of tissue at the upper end of the larva (the end
opposite the protozooid). More zooids are added by budding from
a region between the pneumatophore and protozooid. At all stages
of the life cycle the organism is pelagic and never settles on
the bottom to live a sessile existence.
Swarming
Physalia is at the mercy of the
winds and sometimes entire swarms are blown onto shore. The fact
that some have their sails arrange to tack to the left and some
to tack to the right, means that prevailing winds will be unable
to drive the whole population ashore! Winds can also funnel Physalia into tight-packed
swarms on the ocean's surface that sometimes extend for hundreds
of kilometers. Such aggregation might facilitate fertilization
of the gametes shed into the water, but must also create tight
competition for food and have significant effects on the local
populations of fish and crustaceans in the surface waters. Do Physalia attack and attempt to
sting each other in these aggregates or are they peaceful
affairs?
References
- Note concerning Physalia behaviour at sea; A.H. Woodcock; J. R. Soc. Interface, 2008.
Hydrodynamics of sailing of the Portuguese man-of-war Physalia physalis; G. Iosilevskii and D. Weihs; J. R. Soc. Interface, 2008.- Fine
Structures of the Carbon Monoxide Secreting Tissue in the
Float of Portuguese Man-of-War
(Physalia physalis L.); D.E. Copeland;
Biological Bulletin, Vol. 135, No. 3 (Dec., 1968), pp. 486-500 .
- Colonies
of colonies in Physalia;
P.F.S. Cornelius; In Biology and systematics of Colonial
organisms, G. Larwood and B.R. Rosen (eds)
1979; Academic Press.
Siphonophore Taxonomy
Siphonophorescan be divided into three main groups: the Calycophora, the Cystonecta and the Physonecta.
Physonectids
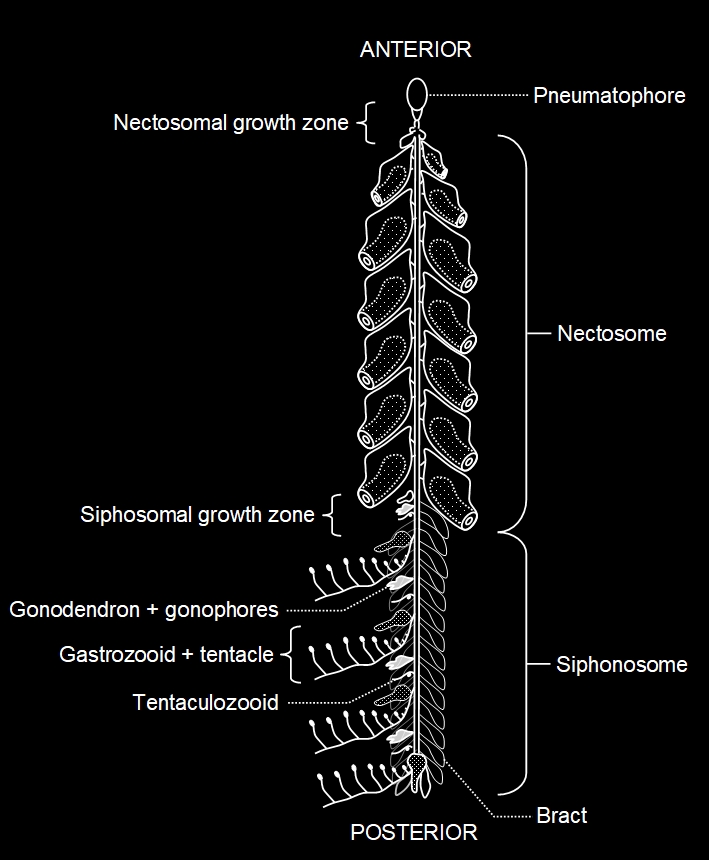
The physonectid siphonophores consist of a small pneumatophore float at the apex (which lacks a pore) from which hangs the stem. The upper part of the stem comprises a column of swimming bells (nectophores) called the nectosome. Below the nectosome the stem bears cormidia and is called the siphonosome. in some modified forms, however, the siphonosome may be shortened and in some the swimming bells may be lost.
Above: a computer model of a siphonophore, similar to Agalma. In this type of siphonophore the zooids bud from a long stalk or coenosarc, which in some forms, like Physalia, is reduced to a flattened disc. There are various types of zooid, each specialised to perform a particular function. At the top is the first zooid to form, which in this case acts as a float (and is probably a modified medusa). Below this are swimming zooids or nectophores which pulse, jetting out water, in much the same way that free-living medusae do. There are six nectophores in this model. Below this is a pair of narrow tubular tentaculozooids, each armed with a single tentacle and several tubular feeding gastrozooids, each again armed by a tentacle and equipped with a mouth. These tentacles will be equipped with clusters or batteries of cells called nematocytes. Each nematocyte contains an organelle called a nematocyst. The nematocyst is a vessel containing a much coiled microscopic 'harpoon', generally poisonous (though in some coelenterates some nematocysts may simply be sticky) which discharges under pressure. In those forms in which the nematocyst harpoons can pierce human skin, the injected venom will cause the jelly's sting. leaf-like zooids called bracts are also present. A bunch of male gonophores can be seen toward the top, with their spermatocyte cargo depicted in yellow, and a bunch of female gonophores lower down, with their eggs shown in red. The gonophores are modified sexual medusae that remain attached in most forms, although some types have free-swimming medusae (sometimes only the female medusae) which do not feed, but degenerate after shedding their gametes. The gametes are shed into the sea and after fertilisation they give rise to ciliated planula larvae which develop into a new primary zooid which produces a new colony by asexual budding.
The
stem (coenosarc) buds off new zooids. It contains thick
mesogloea and has internal radiating septa bearing muscle fibers
and is elastic and muscular. The gastrovascular canal of the
stem is continuous with the gastrovascular cavities of the
zooids and ends in the gastrovascular cavity of the float.
Siphonophores come in a fantastic diversity of forms and many are bioluminescent. They are extraordinary creatures in which incomplete asexual reproduction by budding gives rise to an integrated colony in which all the zooids remain connected via gastrovascular canals that ultimately connect to the stomachs of the feeding gastrozooids.
Above and below: a physonectid siphonophore (based on Dunn and Wagner, 2006). Note the two growth zones: the nectosomal growth zone beneath the float, where new nectophores are added and the siphosomal growth zone where new cormidia are added. The stem of siphonophores bears the zooids on one side, termed the ventral surface, but secondary twisting of the stem may make it appear as the zooids are in spirals or whorls.
Benthic Siphonophores
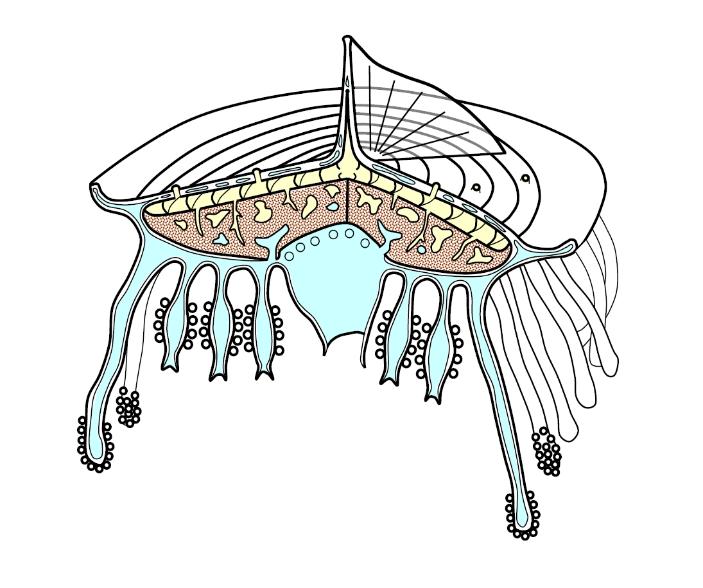
Benthic siphonophoreslive on or near the ocean bottom at a range of depths. The most well studied of these forms are the rhodaliids (family: Rhodaliidae) a group of physonectids. These curious siphonophores have rounded bodies which often hover above the sea-bed anchored by their long tentacles, like tethered hot-air balloons. An example is Stephalia, illustrated below:
Above: the rhodaliid Stephalia (based on Haeckel, 1888 as illustrated in Hyman, 1940: The Invertebrates - Protozoa through Ctenophora). At the top is a large float (pneumatophore) and below this the nectosome and siphosome are compressed into a globular corm bearing a ring or corona of swimming nectophores just below the float and spiral whorls of cormidia: gastrozooids and their tentacles and a main gastrozooid (derived from the larval gastrozooid or protozooid) beneath. Palpons (or gonopalpons), bracts and gonodendra also occur. Notice the single aurophore in amongst the nectophore ring, with its downwardly projected pore. The aurophore is an extension of the float containing additional gas gland tissue. The aurophore defines the dorsal position and the nectophores on either side of it are the oldest. Opposite the aurophore, on the ventral side, lie the two budding zones of the physonectid close together, budding off nectophores and cormidia on either side.
The corm body is hollow in some forms, solid in others. solid corms are gelatinous and contain an anatomizing network of gastrovascular canals. The float forms from an invagination of apical tissue (stem or corm) and is a double-walled structure, each wall consisting of ectoderm and endoderm separated by a thin layer of mesogloea. The main cavity of the float is lined and strengthened by chitin secreted by the ectoderm. Floats may have an apical pore or a basal pore and at least the apical pore has been shown to excrete gas so as to regulate the amount of buoyancy. Some siphonophores make diurnal migrations up and down the water column, but in benthic forms the buoyancy is carefully regulated to maintain the right depth. The gas inside siphonophore floats is similar in composition to air: it is mostly nitrogen with about 15% of oxygen and 1% argon and can be high in carbon monoxide. There is some uncertainty in the literature whether or not the aurophore pore excretes gas (and hence connects to the gas chamber) or whether it connects to the gastrovascular system and so excretes fluid from there.
Another example of a benthic rhodaliid is Archangelopsis
which has more than one type of gastrozooid. The gastrozooids bear
long extensible tentacles with a row of cnidobands on extensible
pedicels, each cnidoblast bears a terminal and extensible tentillum.
Siphonophores with this type of stinging apparatus possess an
interesting prey capture mechanism: the tentillum entagles small
prey, such as small crustaceans, and then retract to bring the prey
closer to the cnidoband. The prey pulls against the tentillum which,
perhaps aided by muscular action of the siphonophore, triggers an
apparent elastic mechanism in which a special strip or helix
of mesogloea releases stores energy and explosively unfolds to hit
the prey with the stinging cnidoband. Fluorescent or bioluminescent
fishing lures on the tentilla may lure prey in.
Calycophorans
The calycophoran siphonophores have no float and no dactylozooids but do have swimming bells. The stem emerges from one bell and is ensheathed at its base by an extension of the adjacent bell called the hydroecium. each cormidium usually consists of a bract, a gastrozooid armed with a tentacle, and one or more gonophores of the same sex which also serve as secondary swimming bells. New cormidia form at a growth zone at the apex of the stem and may detach (and are then known as eudoxomes or eudoxids which have been mistaken as distinct species of siphonophore. The gonophores are not freed. An example of a calycophoran is Lilyopsis, which contains some 27 species. The nectophores occur at the anterior end of a very long siphonophore with hundreds of cormidia. Siphonophores of this type are 'sit and wait' predators: the stem relaxes and the tentacles hang down in a feeding curtain.
Above and below: Lilyopsis (based on Haddock et al. 2005, simplified). Note that the two nectophores are bilaterally flattened and, as is typical in such nectophores, two of the radial canals are modified and sinuous. The somatocyst is a blind-ending extension (diverticulum) of the gastrovascular cavity, which is continuous with the gastrovascular cavity of the stem and zooids. In the bracts of some calycophorans the somatocyst is present as a swollen phyllocyst and is thought to function in food storage and/or as an aid to bouyancy.
The cystonectids, including Physalia, have a single large float and the stem is shortened to a budding coenosarc on the ventral surface of the float. Some of the dactylozooids are very large and armed with long tentacles, other dactylozzoids are smaller. The gastrozzoids hang down in bunches and lack tentacles. Branched gonodendra bear gonopalpons and clusters of gonophores and 'gelatinous zooids' (modified bracts?). The female gonophores are medusoid and may detach; the male gonophores are reduced.
Siphonophores have been difficult to study and still relatively little is known about them and new species are still being discovered, and I suspect many more await discovery.
Velella
(By-the-wind Sailor)
This
creature was once classed as a siphonophore, but is now usually
placed in a separate group. It resembles a siphonophore with a
disc-shaped coenosarc up to 7 or 8 cm across. The coenosarc is
filled with branching channels (presumably filled with water
below and air above) that are lined by photosynthetic zooxanthellae (microscopic algae)
which tap the energy of sunlight to make food for themselves and
their coelenterate host. Above this structure is the float, with
chitin reinforced walls, which contains concentric air-filled
rings called air
tubes.
Each air tube opens to the air via two air
pores
on top of the float. On top of the float is a chitinous
triangular sail. In plan view the creature is somewhat oval, but
wider at one end and angular to form a rounded-rhomboid shape.
The tentacles of the creature are vividly coloured blue or
purple and hang-down beneath the float. There is an outer-ring
of fishing tentacles (dactylozooids) armed with nematocyst
batteries and several inner rings of gastrozooids and
mouth-bearing gonozooids bearing gonophore buds. The medusa of Velella is about 3 mm in size
and is free-swimming and has four-radial symmetry. In the
center, hanging beneath the float is the large central
gastrozooid, with its large mouth. Eight radial canals connect
the stomach of the gastrozooid with a ring canal in the
periphery of the disc. The sail is angled with respects to the
long-axis of the elliptical/rhomboidal float. The sail is
designed to impart stability, and has a low center of
wind-pressure, making it less likely to topple the animal, and
indeed at wind speeds up to 20 knots the animal is stable, but
wind speeds of 40 knots can cause it to flip over end-over-end.
The animal cannot right itself, though it can tense its muscles,
so as to facilitate righting by wave action. If trapped
upside-down, the animal starts to degenerate after a few hours.
Velella was once regarded as a
colony of zooids, but is now often seen as an individual with
several mouths, on account that it seems to have a single
integrated nervous system. The question remains as to whether it
is an individual that resembles a colony or a colony that has
involved into an individual. Evolution often produces more
complex organisms by repeating modules. For example, plants are
modular organisms and some plants can reproduce in this way,
such as the crack willow tree which sheds twigs and branches
which can establish and become new individuals. Some marine
worms reproduce asexually by budding off new individuals at the
rear, but in some forms the new individuals do not readily
separate and the chain of individuals function as an individual.
Taken to its conclusion this process possibly gave rise to the
segments that make up the body of an earthworm, a fish and a
mammal such as the human being. Each segment retains its
ancestral nervous system, albeit in modified form, with the
ganglia alongside the spinal cord in humans being vestiges of
each segments original 'brain'. In Velella we see another example
of how modules, perhaps originally individuals in their own
right, can become so well integrated as to form the organs of a
new modular organism. This is a familiar pattern in evolution -
the replication of units, followed by their modification to
produce new parts and new complexity.
More References
and Further Reading
Dunn, C.W. and Wagner, G.P. 2006. The evolution of colony-level development in the Siphonophora (Cnidaria:Hydrozoa). Dev Genes Evol 216: 743–754 DOI 10.1007/s00427-006-0101-8.
Haddock, S.H.D., Dunn, C.W. and Pugh, P.R. 2005. A re-examination of siphonophore terminology and morphology, applied to the description of two new prayine species with remarkable bio-optical properties. J. Mar. Bio. Ass. UK 85: 695-707. DOI: https://doi.org/10.1017/S0025315405011616
Manko, M.K., Weydmann, A. and Mapstone, G.M. 2017. A shallow-living Benthic Rhodaliid siphonophore: Citizen science discovery from Papua New Guinea. Zootaxa 4324(1):189 DOI: 10.11646/zootaxa.4324.1.11
Munro, C, Vue, Z, Behringer, R.R. and Dunn, C.W. 2019. Morphology and development of the portuguese man of war, Physalia physalis. Nature: Scientific Reports 9:15522 https://doi.org/10.1038/s41598-019-51842-1
Pugh, P.R. 1983. Benthic Siphonophores: A Review of the family Rhodaliidae (Siphonophora, Physonectae). Phil. Trans. R. Soc. Lond. B 301, 166-300.
Article updated: 6 Sep 2020, 7 Sep 2020