| Parasites
of Plants - Oomycetes |

Above: an oomycete zoospore. The oomycetes (oomycotes, 'egg fungi' or 'water moulds') are protofungi (or as some say 'pseudofungi') and most are aquatic. Unlike fungi which produce immotile spores that are dispersed on the wind, oomycetes produce spores, called zoospores, that require water in which to swim by means of two flagella. Having two flagella these spores are described as biflagellate. In the case of those species which parasitise plants, they may swim in a drop of water on the surface of a leaf. One flagellum is a tinsel flagellum, a flagella with tinsel-like lateral branches, called mastigonemes, and this flagellum pulls the cell through the water and so is directed forwards when swimming. The other flagellum is a smooth whiplash flagellum, which pushes the cell through the water and so is held backwards. The flagella motors undulate from side-to-side, with the tip tracing out circles, as waves of movement pass along their length from the base to the tip. The large spherical structure towards the rear of the zoospore is a lipid droplet, which may act as a both an aid to buoyancy and a fuel/energy store.
Oomycetes
(protofungi)
Prerequisites:
fungi.
We begin with a general discussion of the oomycetes. One of the
best studied examples, Saprolegnia, is not a plant
parasite at all, but grows on rotting organic matter, such as
dead fish or dead seeds floating in ponds and the like. Some
species are also parasitic, growing on the scale or eggs of
fish, and also on amphibians, rotifers, nematodes, arthropods and
diatoms, depending on species. This water mould grows as a
mycelium of multinucleate threads, called hyphae (see fungi)
which are branched (and lack internal cross-walls so that the
protoplasm is continuous throughout their length). The mycelium
absorbs nutrients from the surrounding water and the substrate
on which it is growing, that is it is a saprobe (or saprotroph).
The plasma membrane of the hyphae is surrounded by a rigid wall,
which in at least some oomycetes
contains cellulose, a polymer of glucose
(whereas in true fungi the wall contains chitosan, a chitin-like
polymer). [See carbohydrates for an introduction to sugar-based
biopolymers like cellulose and chitin]. The hyphae penetrate the
substrate on which the oomycete is feeding, whilst some hyphal
branches stand out into the water. An internal cross-wall may
develop in these hyphal branches, separating off a large portion
of the tip as a separate compartment. This terminal compartment
enlarges and becomes a zoosporangium, with zoospores developing
inside it. When mature, the zoospores escape through a pore
(ostium) that forms in the tip of the zoosporangium, as shown
below:
Above:
a sporulating zoosporangium. Note the cross-wall dividing the
zoosporangium from its parent hypha. The zoospores escape by
swimming backwards, and then disperse by normal forwards
swimming.
Click images to enlarge.
This is a process of asexual reproduction. After swimming around for a few minutes (immediately on leaving the zoosporangium in some species) the zoospores withdraw their flagella, round-up and then secrete a 'shell' or cyst around themselves. Presumably they may survive harsh conditions that may occur whilst so encysted (such as drying of the pond). In good conditions, however, the cyst soon hatches, and the zoospore re-emerges, only it has metamorphosed into a type which is bean-shaped and with the two flagella inserted midway along the concave side of the cell body. Such a zoospore is shown below.
This
phenomenon, of having two different zoospore types is called diplanetism, and we could call the
second type of zoospore a diplanospore, or secondary zoospore,
to avoid confusion. The reason for this change is uncertain,
though it seems likely that the first arrangement of flagella
assisted the zoospore in reversing out of the zoosporangium,
whilst the second arrangement is perhaps better suited to
dispersal. The second zoospore type swims around for several
hours, so is clearly the main dispersive stage. However, some
oomycetes have monomorphic zoospores, in which only one type of
zoospore, the secondary bean-shaped type, is produced directly
in the zoosporangium. The more complicated version,, involving
two spore types (dimorphic zoospores) may be a relic of some
past evolutionary form, though such 'relics' are typically
retained when they perform a useful function.
Eventually the diplanospore withdraws its flagella, rounds-up
and encysts again. This secondary cyst may emerge as another
diplanospore, or it may emerge as a germinating hypha. In either
case, the final cyst will eventually germinate as a growing
hypha, called a germ tube, which grows from the cyst, feeds and
eventually develops into a new mycelium.
In
addition to this mode of asexual
reproduction,
oomycetes such as Saprolegnia, at some point
undergo sexual
reproduction.
Certain hyphae growing from the substratum produce side-branches
that develop female organs or oogonia (singular oogonium) at
their tips. The oogonium is typically divided from the hyphal
branch supporting it by a cross-wall. The tips of branching male
hyphae also develop end compartments, partitioned off from the
rest of the hypha by a cross-wall, called antheridia (singular
antheridium). Oomycetes are oogamous, meaning that the egg cells
are immotile and larger than the sperm. Each oogonium contains
several such eggs or ova (singular ovum). Each antheridium
contains several male nuclei. The female hyphae release a
hormone called antheridiol, which stimulates the development of
male antheridial branches, and conversely male hyphae release a
hormone called oogonial which induces oogonia development in the
female hyphae.
The male hyphae are attracted to the oogonia, and when an
antheridium makes contact with the outer surface of an oogonium,
it grows one or more hyphae into the oogonium toward one or more
eggs. Male nuclei then travel down these fertilization
hyphae
to the eggs and one male nucleus fertilizes each egg. (The tip
of each fertilization hypha ruptures when it contacts an egg to
allow the sperm to reach the egg). Typically, several antheridia
will fertilize eggs in the same oogonium, though each egg is
only fertilized once. The male and female nuclei fuse to form a
diploid nucleus. (Since the parent mycelia were diploid, it
seems that meiosis occurs in the formation of the eggs and male
nuclei, as these germ nuclei are haploid). The fertilized egg
then secretes a shell or cyst around itself, becoming a resting
oospore.
In
some species some oospores are also formed without being
fertilized by male nuclei. In either case, after a period of
rest the oospores, whilst still inside the oogonium, germinate.
A hypha, called a germ tube, grows out from the oospore,
punctures the oogonium wall and grows out, forming a club-shaped
tip, which eventually becomes compartmentalised by the formation
of a cross-wall at its base. The end compartment develops into a
germsporangium, which produces germ zoospores (which are
diploid) which are released when ripe and disperse and
eventually encyst and germinate to produce new mycelia.
Above:
a sporulating germsporangium. Click image to enlarge.
Special Ability - Electrotaxis
Oomycete zoospores swim backwards to escape the sporangium, but how do they know to do this? Although there may be hydrodynamic factors, such as the confined space of the sporangium and the fact that it appears as if the sporangium also helps squeeze out the zoospores under pressure, it has been suggested that the zoospores align with an electric field generated by the sporangium. Certainly, applying an electric field to the sporangium realigns the zoospores and disrupts the process. This electrotaxis: aligning with and moving down an electrical gradient is a truly fascinating ability.
Furthermore, when infecting plant roots, zoospores accumulate preferentially at specific segments of the root, reflecting the nature of ion pumping that occurs across this part of the root. Roots expend energy in pumping ions in and out, between the root and the soil, with the net effect of taking up mineral nutrients from the soil. For example, protons (hydrogen ions) are pumped out and then taken back in along with nitrate ions. This flow of electric charge varies from one part of the root to another depending on the types of ion pumping taking place in that region. Some species of parasitic oomycete are positively electrotactic and home in on regions of the roots generating a positive electric field, whereas others are negatively electrotactic, homing in on regions generating a negative electric field.
Each zoospore acts like an electric dipole: it has a positive pole and a negative pole. oomycetes are also heterokonts, meaning they have two flagella that are distinctly morphologically different. The zoospores swim with the tinselated flagellum pointing forwards and the smooth flagellum directed backwards. The smooth flagellum possibly acts to steer the cell as well as to push it along. application of electric fields will cause both flagella to turn, redirecting the cell and it has been hypothesized that one flagellum is positively charged, the other negatively charged. For example, the zoospores of Phytophthora palmivora are positively electrotactis and appear to have negatively charged tinselated flagella and positively charged smooth flagella. in contrast, Pythium aphanidermatum is negatively electrotactic and has a positively charged tinselated flagellum and a negatively charged smooth flagellum. (See: Morris, B.M. and Gow, N.A.R. 1993. Mechanism of electrotaxis of zoospores of phytopathogenic fungi. Phytopathology 83(8): 877-882.)
Oomycetes
have helped shape human history!
Some
oomycetes have adapted to life on land by parasitising plants,
forming so-called 'downy mildews'. One species, incidentally, Pythium insidiosum, is a parasite of
mammals, causing disease in horses. Peronospora causes the blue mold
disease of tobacco, Albugo
candida
causes the white rust disease of mustard. Plasmopara
viticola
causes downy mildew of grapes, which almost destroyed the French
wine industry in the 1870's (by accident the first fungicide,
the Bordeaux mixture, consisting of lime and copper sulphate,
was found to treat this disease).
We discuss two groups of oomycetes in this article: the
Saprolegniales and the Peronosporales. The Saprolegniales
include Saprolegnia which we have already
considered in detail. The Peronosporales include Peronospora and Phytophthora species. These plant
parasites grow inside leaves of the host plant, forming a
mycelium in the intercellular air spaces within the leaf
mesophyll (see leaves). It puts out
protuberances, called haustoria, that penetrate the
plant's cells to take nourishment from them. The mycelium then
puts-out aerial hyphae through the stomata of the leaf. These
hyphae, called sporangiophores, produce air-born
spores which may land on an uninfected leaf and germinate in a
drop of water or dew on the leaf surface. When germinating they
put out a hyphal thread, called a germ tube, which locates a
stoma (possibly by touch and/or chemosensation, perhaps
detecting carbon dioxide emissions from the stoma) and then
enters the leaf through the stoma pore and infects the leaf
tissue, forming a new mycelium. [Bordeaux mixture forms
copper(II) hydroxide in water and this kills the germ tubes
which germinate in water].
One of the most influential oomycetes is Phytophthora
infestans,
which caused potato blight, resulting in the Great Irish Potato
Famine of 1845-1852. This tragedy killed some one million people
in Ireland and caused a further million to emigrate. Many
settled in America, shaping the culture of the United states of
America. Phytophthora
infestans
grows by feeding on leaf tissues. Dead spots appear on the
leaves, fringed by growing and feeding mycelium which spreads
out across the leaf, killing it, and puts out sporangiophores
through the stomata of the leaf. These sporangiophores bear
sporangia capsules which break away to be dispersed by the wind.
When they land on a new leaf the sporangia will split open in a
drop of rainwater, releasing swimming zoospores which encyst.
The cyst germinates, putting out a growing germ tube. Sporangia
may also enter the soil, in which the liberated zoospores can
swim through moisture to plant roots, infected the tubers of the
potato plant for example.
A number of species of Phytophthora continue to cause
concern. This oomycete attacks the cambium, the growing part of
tree trunks, roots and branches, responsible for secondary
growth which causes thickening in these structures. Phytophthora infection produces
rusty-red exudations on the bark and sometimes killing the tree.
For example, the 'ink disease' of sweet-chestnut trees kills the
roots and hence sometimes the tree. It favors waterlogged soils
(presumably as this aids zoospore dispersal). A similar disease
occurs in alders; Phytophthora
quercina
attacks oaks and another species attacks the horsechestnut.
Swamp Cancer
Although most oomycetes feed either on dead organic matter (e.g. in ponds) or parasitise plants (such as downy mildew on grapes), some parasitise fish and other aquatic animals, whilst Pythium insidiosum parasitises mammals, such as horses, and occasionally humans. The zoospores are attracted to damaged animal tissues and animal hair as well as the leaves of plants such as lillies and grasses. The zoospores will infect plant leaves in vitro and it is thought that the organism requires a plant as a primary host to complete its life-cycle, but animals may function as secondary hosts when available. Working in rice paddies is a significant risk factor to contracting Pythiosis.
In humans and other mammals, Pythium insidiosum primarily causes a subcutaneous infection, Pythiosis, causing granulomatous lesions in which balls of mycelia grow rapidly underneath the skin, eventually errupting to be deposited on the ground (where released zoospores are thought to infect new plants such that the animal host acts as a means of dispersal). These lesions gave the disease a common name of ‘swamp cancer’. In severe cases the oomycete reaches the bloodstream and may be life-threatening! Less frequently, Pythium insidiosum may also cause intestinal disease. (Phillips, A.J., V. L. Anderson, E. J. Robertson, C. J. Secombes and P. V. west, 2008. New insights into animal pathogenic oomycetes. Trends in Microbiology 16: 13 – 19.)
Oomycete
Parasites of Animals
Oomycetes are important parasites of fish, making them of
considerable economic importance. They are also important parasites
of amphibians. About one-third of amphibian species, globally, are
currently threatened with extinction. There are likely multiple
reasons for this worrying decline and one such factor are oomycetes.
Just as globalization has made it easier for diseases to spread to
ash trees and to humans (e.g. SARS-CoV-2) so it has also exposed
amphibian populations to new diseases in previously pristine
environments. Amphibian populations may have no resistance to a new
pathogen and the consequences can be devastating. In 2007, the
oomycete Saprolegnia diclina was one of several oomycete
species responsible for an emerging disease of amphibians, infecting
embryos of Epidalea calamita (Bufo calamita, the
Natterjack Toad).
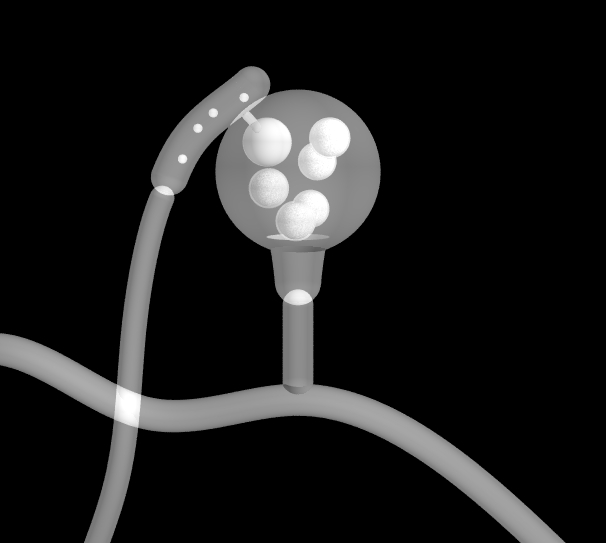
Above: a chain of oogonia of Saprolegnia diclina (rather like a tiny string of pearls).(Based on: Coker, W.C. 1923. The Saprolegniaceae). Oogonia of this species may also occur singly, either on the ends of hyphae or part-way along a hypha (intercalary). Oomycetes form a beautiful diversity of forms.
Saprolegniaceae also produce gemmae (chlamydospores): structures formed from inflated segments or tips of hyphae which become packed with dense cytoplasm and are separated from the rest of the hypha by cross-walls. they may form singly or in chains. Gemmae persist for a long time but are not resistant to dehydration. They are formed under certain (unfavorable) environmental conditions and under more suitable conditions may germinate via a germ tube, or release zoospores or act as a female gametangium (oogonium). In S. diclina the gemmae, produced singly or in chains, are either long and pointed or knotted structures that germinate to release zoospores. The eggs are resistant to drying.
Even fleas have fleas
Many oomycetes are parasites, though by no means all, and some specialise in parasitising other oomycetes. Olpidiopsis saprolegniae infects Saprolegnia, forming its sporangia inside the host hyphae either at the tips but more often intercalary (at some point along the hyphal length) or sometimes it may infect the oogonia or sporangia of its host. Infection causes the host hypha to swell at the infected region, forming a gall containing the sporangia of the parasite. Wherever it is situated, its sporangia grow out one or more tubes until they pierce the host wall and open through the outside. The numerous very small zoospores escape through these tubes. These zoospores are bean-shaped with a pair of lateral flagella (secondary-type zoospores). Numerous zoospores (which are thus necessarily small) increases the odds that one of them will locate a suitable host. Thus, Olpidiopsis is an endoparasite - living within the cells of its host. Forms of Olpidiopsis also infect red algae, and so may be of major economic importance.
The parasite also develops oogonia within the host gall. Each oogonium is accompanied by one (to three) antheridial cells. The antheridial cells appear empty once the eggs are mature (since their protoplasts enter the oogonia to fertilize the eggs). Each oogonium contains only one to two eggs, and each oogonium is surrounded by a warty or spiny wall.
Above: Olpidiopsis saprolegniae. A. A gall containing several developing sporangia; B. a gall containing several sporangia and an oogonium with accompanying antheridium; C. zoospores; D. sporangia growing on a host oogonium.
Further examples of Oomycetes
The classic life-cycle, complete with diplanetic zoospores is found in the oomycete genus Saprolegnia, which occurs in soil and fresh-water. S. ferax and S. parasitica are parasites of fish and fish eggs and of considerable economic importance. Saprolegnia also has a tendency to develop a new sporangium inside the empty hull of the previous sporangium, so that a sequence of nested sporangia may form, rather like Russian dolls, with only the inner one ripe and bearing spores. However, this life-cycle is extensively modified in other forms.
Achlya occurs in soil and on waterlogged plant debris (such as twigs). The primary zoospores do not generally disperse far, but cluster together in a hollow ball at the mouth of the sporangium, where they encyst. When the cysts germinate, a secondary zoospore emerges through a pore in the cyst wall and swims about for a while before it too encysts. Each of these secondary cysts germinates to form either a germination tube or another secondary-type zoospore (i.e. bean-shaped with lateral flagella). The cysts can persist for two months at low temperature and so presumably represent an overwintering stage.
Aphanomyces forms narrow, delicate hyphae on the empty molted shells of arthropods in water. A. astaci is a parasite of crayfish, whilst A. euteiches infects the roots of pea plants and has no flagella on its primary spores, so produces only one type of zoospore, the secondary type.
Thraustotheca clavata has no free-swimming primary zoospores and the spores encyst within the sporangium which ruptures in an irregular manner to release the cysts which upon germination produce secondary zoospores (bean-shaped with lateral flagella) which swim about before themselves encysting and these cysts germinate either as an emerging germ tube or another secondary-type zoospore. Such an oomycete is described as monomorphic (only one morphological type of zoospore) but polyplanetic (producing several generations of these zoospores).
Dictyuchus has no free-swimming primary zoospore and the sporangium may detach and continue forming zoospores which encyst within the sporangium. Each germinates via a separate pore in the sporangium wall to release a secondary zoospore which swims around before encysting and later germinating in favorable conditions.
Aplanetic forms (forms producing no motile zoospores) may occur in several genera under certain culture conditions. The non-motile spores encyst within the sporangium and upon germination they put out germ tubes that penetrate the sporangium wall.
The Peronosporales are a group of parasitic oomycetes, either obligate parasites, or forms that feed parasitically when given the opportunity and then feed on the dead remains of the host they kill. Pythium is a saprophyte, feeding off dead organic matter in water and soil but also an opportunistic (facultative) parasite on crowded seedlings growing in waterlogged conditions, e.g. cress seedlings (Lepidium sativum). it secretes enzymes that dissolve the biological glue holding the host's cells together (and possibly also toxins that kill host cells). The sporangium develops a protuberance which exudes a thin-walled vesicle containing the developing zoospores. sexual reproduction gives rise to oospores.
Phytophthora is another of the Peronosporales and a genus of economically important plant parasites (mainly obligate parasites). For example, P. infestans is a causative agent of potato blight, P. cinnamomi infects woody plants and P. fragariae causes red-core disease in strawberries. The mycelium is intercellular and produces haustoria to infect host cells. Aerial sporangiophores, e.g. growing out through a leaf's stomata, generate pear-shaped sporangia at their tips which detach to be dispersed by the wind (in terrestrial forms). Germination of the spores may be by indirect germination, giving rise to zoospores, or direct germination to produce a germ tube. This germination 'choice' seems to be influenced by temperature: temperatures below 15oC induce indirect germination in P. infestans, whilst temperatures above 20oC induce direct germination. sexual reproduction produces oospores.
Article updated: 10/8/14, 19/12/20
Article last updated: 21/12/20
Some negative images for printing: click images for full size.